QA Consulting often advises companies to generate quality plans to ensure that projects are implemented in an organized and comprehensive manner. In addition to serving as objective planning tools, another inherent advantage of quality plans experienced by some companies has been the avoidance of 483 observations.
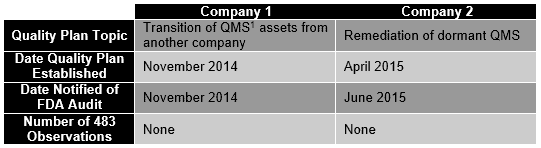
Illustrated below are two companies QA Consulting recently worked with to generate quality plans, which subsequently were essential to their successful FDA inspections.
For both companies above, the agency’s investigators specifically noted that because the quality plans were established prior to announcing the inspection, the existing QMS gaps addressed within the quality plan did not warrant observations.

Assess your organization and determine if there is a need for quality planning for any gaps or complex activities. Quality plans may also address independent management activities such as a facility move, remediation to comply with updated standards, and implementing unique device identification (UDI).
QA Consulting recommends leveraging the guidance for generating quality plans provided in ISO 10005:2005 for to ensure planning is thoroughly carried out.
If you need assistance with quality planning, please call 512-328-9404 or contact us at info@qaconsultinginc.com.