Author: Anne Holland, CEO and Founder
CEOs can be demanding, I should know.
Often, instead of working alongside their quality and regulatory team to set a realistic go-to-market timeline, your CEO will let you know they wanted it done yesterday, and then ask how long will it take and how much does it cost.
To make your job easier, I have outlined five steps that will help you work more collaboratively with your CEO to embrace a quality-first approach to bringing your first medical device to market.
I encourage you to coach your leadership team to see industry standards and regulations as methods for bringing products to market safely and efficiently, instead of viewing them as hurdles to overcome.
While tempting, cutting corners isn’t likely to get your new medical device to market faster; and in fact, increases the likelihood of delays due to non-compliance and could result in lost revenue or may even result in an unsafe product.
Putting the time and effort into dotting the i’s and crossing the t’s will pay dividends in the long run. It may even result in getting your medical device to market before your CEO expects it.
Step 1. Establish Expectations
First things first, your CEO needs to know that to get your company’s new medical device to market, you need a comprehensive quality and regulatory compliance strategy.
You’re not just putting together a spreadsheet of action items or marking off tasks on a checklist of activities; you are looking at the big picture.
To start, you need a state of the art design foundation built in conjunction with your Quality Management System (QMS). Your team will then use the QMS to develop a decision-making framework with short-term and long-range goals, including the actions and resources needed to reach each milestone.
In return for management’s buy-in, you must commit to constant communication to keep your CEO abreast of the project status. Weekly project updates, either in-person or summarized via email, are a must. Some CEOs, myself included, tend to fill in information gaps with negative assumptions. If they haven’t heard from you, they’re in the dark and may assume that nothing is happening, or worse, that something is wrong.
Step 2. Educate Your Stakeholders
As they say, knowing is half the battle. Make sure your leadership team has a basic knowledge of the following:
- FDA’s medical device regulations and associated timelines
- Relevant industry standards
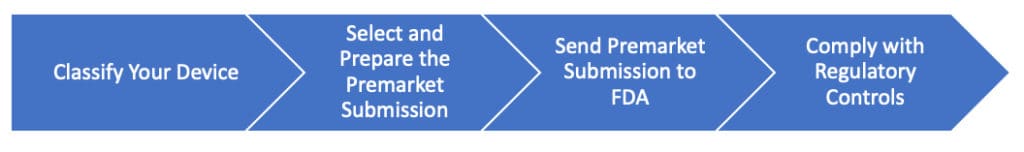
If you’re planning to sell or distribute your medical device in the United States, begin with understanding the FDA’s four premarket requirements.
For those planning to sell or distribute a new medical device in the European Union, reference the EU Medical Device Regulation (MDR) as well as ISO 13485.
Step 3. Accelerate Expectations and Map Your Journey to Launch
Now that your CEO knows what to expect and has a solid understanding of applicable regulations and industry standards, it’s time for the design and development planning to get your medical device to market safely and efficiently.
Working alongside your CEO to articulate your company’s goals, your role is to ensure that compliance requirements are integrated into a planning process that will help you reach those goals. Sharing the design plan that outlines the goals, objectives, interfaces, responsibilities, and schedules gives your CEO the information they need to understand the scope of the project and where your team is in the process.
Additionally, strong design control adherence ensures that product development runs smoothly and reacts nimbly to any changes that arise.
Step 4. Anticipate Potential Risks
Your CEO should also understand the critical importance of identifying and mitigating any potential risks. Robust risk management planning, analysis and mitigation efforts throughout product development can be a life-saver. When a problem arises, you will have already planned for how to deal with the situation.
Further, an ISO 14971-compliant risk-benefit profile based on your company’s risk tolerance will put your CEO’s mind at ease. This level of advanced planning is another opportunity for you to avoid delays in getting your product to market promptly.
Learn more about the recent updates to medical device risk management standards.
Step 5. Execute and Optimize Your Strategy
Once you have a defined project plan in place, it’s time to execute and optimize your quality-first strategy.
This is where the rubber meets the road. Your design starts with a design plan and must be tested through design verification and validation. Once testing demonstrates that your device is safe and effective, it is time for design transfer (launching production) and process validation. The output of your product must be consistent and reliably achieved.
Help Your CEO Sleep Better at Night
Your CEO has a lot to think about at night. Don’t let quality and regulatory compliance issues be what keeps them up. With the right outsourced partner who understands medical device quality systems and medical device regulatory affairs consulting, you can get your product to market safely and on your CEO’s timeline.
Contact us today to discuss taking a quality-first approach to bringing your medical device to market.