QA Consulting has identified a shift in perception within the medical device industry regarding FDA inspections in recent years. Specifically, manufacturers find that the agency’s investigators are fairer in their inspections and more tolerant of gaps when manufacturers demonstrate a proactive commitment to compliance and are actively working to remediate any gaps.
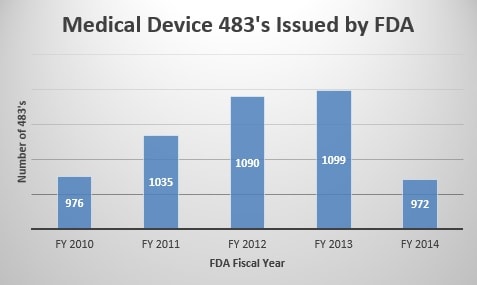
The FDA’s data on 483’s from 2014 mirror the industry’s sentiment. As Anne Wilson shared in the Current Trends in FDA Inspections presentation, she noted that medical device 483 and Warning Letter issuances decreased by 12% and 16%, respectively, from 2013 to 2014[1]. In fact, medical device 483’s issued by the agency in 2014 were the fewest amount over the past five fiscal years, at 972.
Another way in which manufacturers are experiencing a refreshing shift from the FDA is the issuance of Untitled Letters in lieu of Warning Letters when regulatory violations relate to advertising and promotion. An Untitled Letter is defined by the FDA as “… an initial correspondence with regulated industry that cites violations that do not meet the threshold of regulatory significance for a Warning Letter. CDRH issues advertising and promotion Untitled Letters to companies that commercially distribute medical devices.”[2] When an Untitled Letter is issued, the FDA collaborates with the manufacturer to resolve any issues.
Although there may be obvious weaknesses in a manufacturer’s QMS, a warning letter is not always imminent for every inspection. The trends described above speak to the FDA/manufacturer relationship as becoming more collaborative rather than authoritative.
If you need assistance with preparing for or responding to an FDA inspection, please call 512-328-9404 or contact us at info@qaconsultinginc.com.